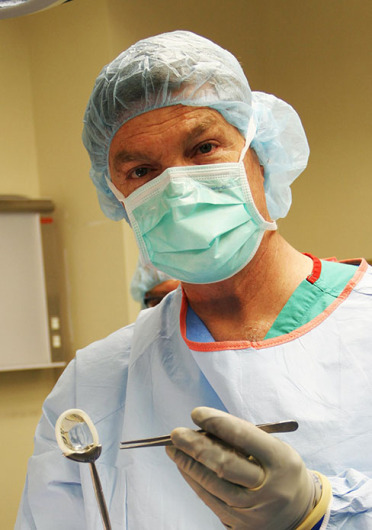
Christopher Kaeding, the executive director of Ohio State’s Sports Medicine, was recently the first surgeon in the U.S. to implant a new plastic meniscus device. Credit: Courtesy of Wexner Medical Center
Christopher Kaeding, the executive director of Ohio State’s Sports Medicine, was recently the first surgeon in the U.S. to implant a new plastic meniscus device for patients suffering from persistent knee pain because of meniscus injury. That device could help patients avoid knee replacement years later.
The “NUsurface” implant, manufactured by Active Implants, a Tennessee-based company specializing in orthopaedic implants, is currently being tested in an FDA-approved multicenter, randomized, control trial at seven locations in the U.S., including the Wexner Medical Center. The surgery was performed in Columbus on Jan. 21.
The implant has already been “successfully completed (in) a multicenter study in patients in Europe and Israel,” according to the study website, adding that, “since 2008, the NUsurface Meniscus Implant has been in use in Europe.”
“The meniscus functions as a protective pad — it helps decrease peak contact forces across the knee, it helps add stability to the knee,” said Kaeding, who is also head team physician for the OSU athletic department and a professor in the Department of Orthopaedics.
“It is not uncommon to tear the meniscus … When you tear a meniscus, most of them unfortunately don’t have a capacity to heal,” he said.
Those tears can then lead to arthritis in the knee, which could in turn make a knee replacement necessary, Kaeding added.
“We hope that this meniscus implant, by putting it into the knee, can restore some of the function of the meniscus, decrease the amount of pain the patient has, and hopefully decrease the progression of any arthritis,” he said.
Currently, there are only few options to treat meniscus injuries. They include anti-inflammatory drugs, physical therapy, injections in the knee or a knee brace. Ideally, Kaeding said, the implant helps delay knee replacements and might even make these surgeries unnecessary.
The implant, made of plastic, is about 1.5 inches wide and 2 inches long, and has the consistency of a pencil eraser. It “can conform to the normal anatomy of the knee,” Kaeding said.
“I’m excited about the technology because it provides a solution, at least an option for these patients that currently don’t have many good options,” Kaeding said. “I’m optimistic that this will be a great addition to what we can offer patients that lost the meniscus.”
The surgery itself takes 1 to 1.5 hours, and the patient can normally leave the hospital the same day. Within a week to 10 days, patients are expected to walk normally, Kaeding said.
Kaeding said 54-year-old Drake Ross, the patient who received the implant at the Wexner Medical Center, “is now two weeks out and doing very well.”
Meniscus injuries are a common condition in the U.S. “Currently, an estimated 720,000 patients undergo knee replacement surgery yearly,” according to a press release by the Wexner Medical Center. “That number is expected to skyrocket to 3.5 million cases by 2030.”
Patients between 30 and 75 years old who have had one part of the meniscus trimmed out in surgery and suffer from knee pain could be eligible for the VENUS Clinical Study, which stands for “Verifying the Effectiveness of the NUsurface System.” Those patients can get in contact with the OSU Sports Medicine Center to determine further criteria of eligibility.
This is a comparative study, though, so only 50 percent of the patients enrolled in the study receive the implant, while the other half are treated with “non-surgical standard of care,” according to the study website.
The study wants to “prove that the NUsurface Meniscus Implant works as well as or better than the current standard of care,” the website states.
The FDA declined to comment because regulations prohibit the agency from discussing any product that has not been approved.
Kaeding said it will be several years at the earliest before the FDA approves the product for regular use.
The OSU Sports Medicine Center has about five ongoing clinical studies, and Kaeding said the Wexner Medical Center in its totality, especially the James Cancer Hospital, has many more ongoing trials.
![Ohio Gov. Mike DeWine signed Senate Bill 1 — a higher education overhaul bill — into law Friday. [Ohio Gov. Mike DeWine speaks at the State of the State address in Columbus. Credit: Joshua Gunter via TNS]](https://www.thelantern.com/files/2025/03/20250204-AMX-US-NEWS-DEWINE-BUDGET-INCREASES-TAXES-FOR-1-PLD-384x253.jpg)

